[PR] Edgecare’s EdgeFlow UH10 Achieves CE Marking Under EU MDR, Driving Global Market Expansion
Page Info.

Main Text
Edgecare’s EdgeFlow UH10 Achieves CE Marking Under EU MDR, Driving Global Market Expansion
Compliance with EU Medical Device Regulation Unlocks European Market Potential
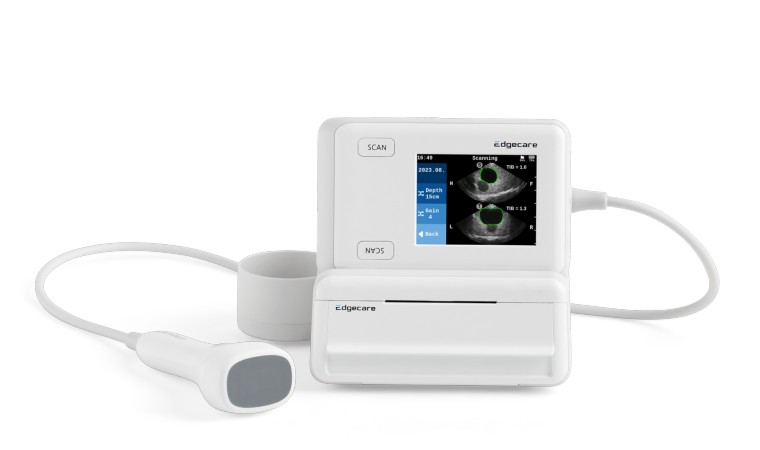
Seoul, South Korea - January 9, 2025 - Edgecare announced on the 9th that its ultrasound bladder scanner, EdgeFlow UH10, has obtained CE marking under the European Union Medical Device Regulation (EU MDR). This certification follows its FDA clearance in March 2024 and represents a significant milestone in the company’s efforts to expand its presence in the European medical device market and enhance its global reach.
The EU MDR, officially known as Regulation (EU) 2017/745, establishes more stringent requirements and processes compared to its predecessor, the MDD. It mandates comprehensive clinical evaluations and robust post-market surveillance, ensuring that compliant products gain not only market access but also increased trust and credibility worldwide.
The EdgeFlow UH10 is a next-generation ultrasound bladder scanner powered by AI-driven deep learning technology. It incorporates DualActive technology, enabling simultaneous high-resolution transverse and sagittal ultrasound imaging via its electronic probe. With advanced features such as LiveContour for real-time bladder localization and MaxContour for automatic calculation of the largest cross-sectional area, the scanner provides precise and rapid volume measurements.
Yangmo Yoo, CEO of Edgecare, stated, “Achieving CE marking under the EU MDR demonstrates the EdgeFlow UH10’s innovation and safety, solidifying its reputation as a reliable bladder monitoring solution for healthcare professionals across Europe and beyond.”
The EdgeFlow UH10’s portability and intuitive interface make it suitable for diverse clinical environments, including hospital examination rooms, wards, rehabilitation centers, and long-term care facilities. Its ease of use enhances workflow efficiency, earning praise from medical professionals globally.
With this achievement, Edgecare aims to reinforce its presence in the European market and broaden its global partnerships. The company plans to expand its European distribution network and enhance visibility through active participation in local conferences and exhibitions.
- Previous[입찰공고] [소재부품장비사업] 주식회사 엣지케어 펄스-에코 시험 장치 24.10.28
- Next[Event] (Jan 27-30) Edgecare at Arab Health 2025 25.01.02