[PR] Edgecare Inc. Receives MFDS Certification for Hemodynamic Monitoring Doppler Ultrasound Device, EdgeFlow CW10
Page Info.

Main Text
Edgecare Inc. Receives MFDS Certification for Hemodynamic Monitoring Doppler Ultrasound Device, EdgeFlow CW10
Seoul, South Korea – August 21, 2024 – Healthcare innovation company Edgecare Inc. (CEO: Yangmo Yoo), which develops and markets ultrasound-based patient monitoring solutions, announced on the 21st that its hemodynamic monitoring Doppler ultrasound device, EdgeFlow CW10, has recently received certification from the Ministry of Food and Drug Safety (MFDS) in South Korea.
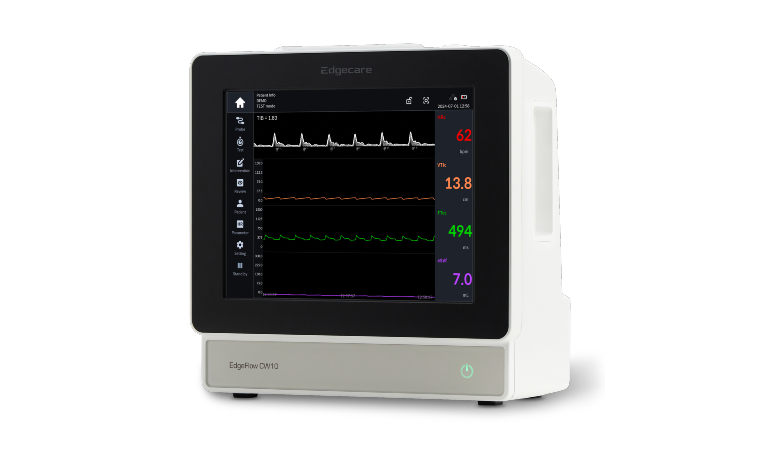
The EdgeFlow CW10 is a medical device that uses a non-invasive, patch-type probe to continuously monitor real-time fluid responsiveness via the carotid artery. It minimizes patient burden while providing real-time blood flow data, helping medical professionals quickly and accurately assess patient conditions. It is particularly essential for patients in intensive care units (ICUs) and operating rooms who require immediate fluid management. Compared to traditional invasive methods, the device significantly improves safety and efficiency, allowing it to be applied to a broader range of patients.
With this MFDS certification, the EdgeFlow CW10 is now ready for full-scale use in Korean medical facilities, signaling a new breakthrough in the domestic medical device market. Yangmo Yoo, CEO of Edgecare, expressed his hopes that "Korean medical professionals will be able to manage critical patients more safely and effectively with the EdgeFlow CW10" and stated that the company "plans to continue introducing innovative products that enhance patient safety and clinical efficiency."
Edgecare will also participate in MEDICA 2024, the world’s largest B2B medical trade fair, held in Düsseldorf, Germany, this November. At the event, Edgecare plans to introduce the EdgeFlow CW10 and showcase its differentiated performance and proprietary technology to healthcare experts and industry leaders from Europe and around the globe.
The introduction of the EdgeFlow CW10 into the Korean market is expected to significantly contribute to rapid decision-making and efficient patient management in the hemodynamic monitoring field, particularly in the treatment of critically ill and acute patients.
- Previous[Event] (Jan 27-30) Edgecare at Arab Health 2025 25.01.02
- Next[Event] (Nov 11-14) Edgecare at MEDICA 2024 24.08.13